Eva Chi, University of California at Berkeley
Evan Knaus, Colorado State University
1997 Summer REU Program at Colorado State University
Advisor: Dr. James Linden
Corporate Sponsor: Richard Stoner, Pres., Aeroponics International,
Berthoud, CO (patented)
August 8, 1997
Earlier studies of mung beans grown in metal trays subjected to a flour slurry gave inconclusive results – the laminarinase activity of treated beans fluctuated daily with respect to control beans.
When multiple bean tissue samples were used to reduce the effect of biological variability and experimental uncertainties, it was found that treated Adzuki beans gave higher specific laminarinase activity in the presence of . Mung beans grown in test tubes subjected to different elicitors gave a significantly higher laminarinase activity on day 10 than day 6. The kinetics of induction by elicitors and the effects of pathogen on treated beans remains a part of future research.
Introduction
During normal germination and growth, crop plants are often attacked by a broad spectrum of pathogenic microorganisms. Traditionally, pesticides and fungicides are used to fight fungal diseases. However, these chemicals are often toxic, non-biodegradable, and species specific. Genetic manipulations are also used to modify plants to secret toxins to fight fungal diseases. Because both methods are undesirable, raising health and environmental concerns, an alternative and environmentally safe pathogen control strategy is therefore needed.
In 1994, an (ODC) system, patented, was developed at Aeroponics International, of Berthoud, Colorado and Dr. Jim Linden, Professor of microbiology Colorado State University. This system takes advantage of a natural defense mechanism in plants. One such defense mechanism is through the production of pathogenesis-related (PR) proteins2, which include ase and b-1,3-glucanase (laminarinase) when the plants come into contact with pathogens. These enzymes hydrolyze polysaccharides found in the cell walls of many fungi2, thus degrade fungal cell walls and lead to cell lysis. ase cleaves the b-1,4 bonds found in ; laminarinase cleaves the b-1,3 bonds found in laminarin. , , and N-acetylchitooligosaccharides with degree of polymerization 6 to 8 were found to induce the expression of ase and laminarinase when they are introduced to plants2. Aeroponics International developed a seed encapsulation technology as a method to apply a constant source of to seed potatoes. During the greenhouse studies conducted at Colorado State University, Michigan State University, and the University of Wyoming it was shown that encapsulated potatoes exhibited an increased ase activity and a decreased susceptibility to fugal diseases.
Planetary missions and long term space explorations require self-contained, regenerable life-support systems, which include plants, to recycle water and waste, and to provide food and oxygen. Thus far, attempts to grow plants aboard the Russian MIR Space Station have been problematic – plants grown are often attacked by pathogens. NASA thus has offered the opportunity to evaluate the organic disease technology aboard the MIR Space Station. Because of the physical constrains of the apparatus, Fluid Delivery Apparatus (FDA), that are to be used to grow plants on MIR, beans will be used. Prior to testing the technology in space, where the effects of elicitors in low-gravity can be assessed, ground-based experiments are to be performed. The objective of the project during the summer is to examine the responses of control and treated beans to different elicitors. Elicited responses will be evaluated via enzyme assays. Because several forms of ase have been reported in a wide variety of crop plants, this project also serves as a start of the study of applying the organic disease control strategy to other major crops.
Plant Materials
Bean sprouts were grown from dried mung and adzuki beans. When
subjected to moist environment, mung and adzuki beans germinated after
three and five days, respectively. Different growth rates and germination
rates were observed with different elicitors. Water and a dilute
solution of calcium chloride (0.18 g/100 ml dH2O) were used as control
growth solution. Elicitors were introduced to bean seeds by growing
bean sprouts in solutions of elicitors and control growth solution.
Root and stem tissue of sprouts, 0.5 g were used to perform each enzyme
assays. Tissue samples were ground using homogenizers; cellular proteins
were extracted using a 0.05 M sodium phosphate buffer at pH 5.5.
Cell debris were removed by centrifugation at 10,000 rpm for 10 min.
Supernatant was filtered through Sephedex G25 gel filtration columns to
separate desired enzymes from sugars and peptides of molecular weight 5000Mr
and smaller.
ase Reducing Sugars Assay
The colorimetric assay tests for the activity of ase in the enzyme extract derived from bean tissue samples. Purified collodial (insoluble) solution was used as the substrate. When is cleaved by ase, a reducing end is left. Dinitrosalicylic acid (DNSA) reagent was used to react with the reducing sugars in solution to give color changes in the visible range (540nm). A double beam spectrophotometer, using water as the reference blank, was used to obtain absorbance readings of DNSA solutions. The amount of reducing sugars was calculated from absorbance verses glucose concentration standard curves.
To correct for the background levels of reducing sugars in enzyme extracts and substrate solutions, blanks of each solution were prepared during each assay to allow the net calculation of the level of reducing sugars resulted only from enzymatic reactions. Each reaction solution contains 1ml of enzyme extract and 10 mg of purified ; each enzyme blank contains 1 ml of enzyme extract; each substrate blank contains 10 mg of . Appropriate amounts of sodium phosphate buffer solution were added to each sample to give a consistent overall volume of 2 ml. Sample solutions were incubated and shaken overnight at 37oC to allow sufficient hydrolysis of the insoluble substrate. Unreacted collodial was removed by centrifugation. Aliquots (0.5ml) of the supernatant solutions were added to 3 ml of DNSA. Reaction mixtures were incubated in boiling water for 10 minutes. Absorbance readings, at 540 nm, were subsequently recorded using a double beam spectrophotometer. Standard curves, using N-acetyl-glucosamine as the reducing sugar, were prepared during each assay.
Because of the high levels of background reducing sugars in both the
substrate and enzyme extract solutions, the sensitivity of the ase
assay was greatly reduced. Absorbance readings for substrate and
enzyme blanks were sufficiently high that the net reaction absorbance calculated
were negative. Another assay, laminarinase assay, was therefore adapted
to replace the ase assay.
Laminarinase Reducing Sugar Assay
This colorimetric assay tests for the activity of b-1,3-glucanase (lamininarinase) in the enzyme extract obtained from bean tissue samples. A soluble laminarin solution was used as the substrate; DNSA reagent was used to react with the reducing sugars in solution. Absorbance readings were recorded at 540 nm using a double beam spectrophotometer with water as the reference blank.
Enzyme and substrate blanks were used to correct for background reducing
sugar levels. Reaction mixtures contain 1 ml of laminarin (10 mg)
solution and 1 ml of enzyme extract; enzyme blanks contain 1 ml of enzyme
extract and 1 ml of buffer; substrate blanks contain 1 ml of laminarin
solution and 1 ml of buffer. Standard solutions were prepared using
a standard glucose solution. Above sample solutions were incubated
in a water bath for 30 minutes at 37oC. 3 ml of DNSA reagent were
added subsequently to all solutions and incubated in boiling water bath
for 10 minutes. Absorbance readings were recorded at the end of incubation.
Protein Assay
The total amount of protein in enzyme extracts was determined using the BCA (bicinchoninic acid) protein assay (product of Pierce Chemicals, ) This assay relies on the biuret reaction1 (protein reducing Cu2+ in alkaline medium to produce Cu1+), and the coordination of BSA to the Cu1+, which gives a purple solution with strong absorbance at 562nm.
Assays were conducted in triplicates. Reaction mixtures were prepared
using 0.1ml of enzyme extract and 2ml of working reagent (50 parts of reagent
A and 1 part of reagent B). Reaction mixtures, along with protein
standard mixtures which uses bovine serum albumin (BSA) as the protein,
were incubated for 30min. Absorbance readings were recorded at the
end of incubation.
Results and Discussion
Data Analysis
Absorbance readings obtained from assays were converted to specific enzyme activities to allow the comparison of control verses treated beans enzyme levels. Such comparison will allow the assessment of the effects of elicitors. One unit of specific enzyme activities is defined as the amount of enzyme required to release 1 mmole of glucose equivalents per minute per milligram total protein present in the enzyme cell extract. Net absorbance due to enzyme activity was calculated by subtracting from the reaction absorbance the enzyme blank absorbance and substrate absorbance. Equivalent glucose concentration (mg/ml) of the net reaction absorbance is calculated from the corresponding standard curve. Reaction rate (mmol / min) is then calculated from incubation time and the molecular weight of glucose. Because of the possible inconsistency introduced from the grinding of tissue samples, some might have disrupted more plant cells to release more protein, specific enzyme activities are more meaningful results. They are obtained by dividing the reaction rate by the total amount of protein present in the solution. The amount of protein present was calculated from absorbance readings collected during the protein assay and the protein standard curves.
Because the final results are reported as differences between control
and elicited specific enzyme activities, meaningful conclusions can only
be drawn after appropriate statistical analysis. However, due to
time constraint and the large amount of data collected, there were no time
to perform such analysis. Results presented thereafter are average
values from triplicate readings.
ase Activity
As described previously, unsatisfactory data were obtained during ase
assays. Absorbance readings of substrate blanks and enzyme blanks
were high, indicating high background levels of reducing sugars.
As a result, negative net reaction absorbances were observed during
assays. Purified colloidal solution was reduced using sodium
borohydride to lower background levels of reducing sugars. No significant
improvement in absorbance readings was observed.
Laminarinase Activity
Laminarinase assays were adopted instead of ase assays to evaluate enzyme activities. Laminarinase are produced by plants through induction following stimulation of barley cells with N-acetylchitooligosaccharides (fragments of ) of fungal cell wall origin2. Because laminarinase and ase belong to the same group of pathogenesis-related proteins, and are induced by the same class of oligosaccharides, an assessment of the level of laminarinase in beans is therefore a valid alternative to ase.
Beans Grown In Trays
Three batches of beans were grown in metal trays.
Each batch used 20 mung or adzuki beans which were placed between moist paper towels. The beans were sterilized using a dilute 10% bleach solution, and growth media, trays, and paper towel were autoclaved prior to germination. Batches 1 and 2 were grown using a elicitor. Laminarinase activities are shown in the following graphs.
Figure 1: Laminarinase Activity of mung and adzuki beans. Batch 1 beans. Water was used as control growth media, and was used as elicitor.
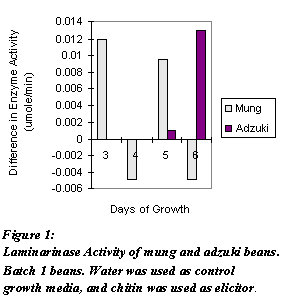
Figure 2: Laminarinase Activity of mung beans. Batch 2 beans. Water was used as control growth media, and was used as elicitor.
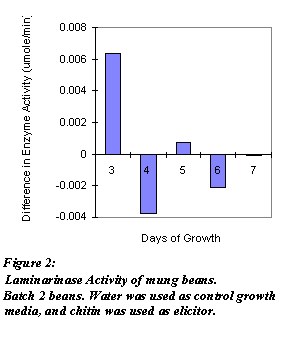
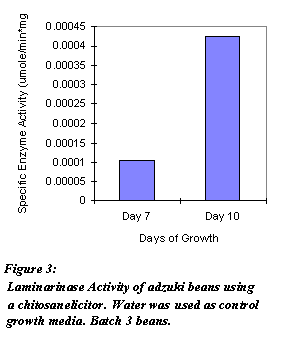
As shown in Figures 1 and 2, enzyme activity fluctuated from day to day. One tissue sample from control and treated sprouts was taken each day. Total protein concentration of the enzyme extract were not determined. Biological variation among the sprouts and error introduced by using enzyme activities, not specific enzyme activities, could have resulted in the fluctuation. From these assays, modifications to future assays are made. Figure 3 shows the specific enzyme activities of adzuki beans grown for Day 7 and Day 10. Three tissue samples from both control and treated sprouts were taken each day. As shown, both days exhibited higher specific enzyme activities.
Figure 3: Laminarinase Activity of adzuki beans using a elicitor. Batch 3 beans. Water was used as control growth media.
Beans Grown in Test Tubes
To better simulate the growth condition aboard the MIR Space Station, mung beans were grown in test tubes, and are subjected to three different elicitors with various concentrations.
Control beans were grown in a dilute solution of calcium chloride. Results
of laminarinase assays conducted on day 6 and day 10 of growth are shown
in Figure 4. Differences in specific enzyme activity found between
elicited and control beans are small and negative on day 6, where as the
differences are significantly larger on day 10.
Figure 4: Differences in the specific enzyme activity of
control and treated beans grown in test tubes. Day 6 and Day 10.
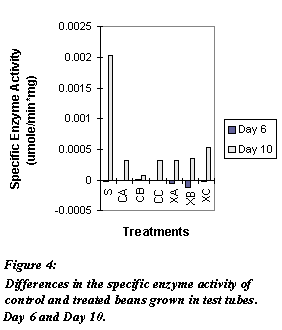
Key: (Note: amounts of each treatment are proprietary thus they are not the provided in this review)
Conclusions
Mung and adzuki beans were used to study the effects of elicitors, which are used to stimulate the production of disease-fighting enzymes, ase and b-1,3-glucanase. Enzyme assays were conducted to evaluated the elicited responses. Because ase assays did not yield usable results, laminarinase assays was used instead.
Studies of the first two batches of mung and adzuki beans grown in metal
trays subjected to a flour slurry gave inconclusive results – the
laminarinase activity of treated beans fluctuated daily with respect to
control beans. To reduce the effect of biological variability and
experimental uncertainties, three bean tissue samples were used during
subsequent assays. Protein correlation, which allowed the calculation
of specific enzyme activity, was also adopted. It was found that
treated Adzuki beans gave higher specific laminarinase activity in the
presence of . Mung beans were grown in test tubes to better simulate
the growth conditions that will be used aboard the Russian MIR Space Station.
These beans were grown using different elicitors. It was found that
treated beans gave a considerably higher laminarinase activity on days
10 than 6.
Recommendations for Further Work
ase activities in potatoes are being studied on a molecular level.
Genes responsible for the expression of various ase are sequenced.
It would be interesting to study weather genes encode for ase among
other crop plants, includes other legumes are homologous to that of potatoes.
With such studies, the applicability of elicitation by oligosaccharides
as a mean of natural and environmentally safe disease control strategy
could be determined to include other crop plants grown here on earth and
in space.
References
1. Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951). Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 193: 265-220.
2. Kaku, H., Shibuya, N., Xu, P., Aryan, A.P. and fincher, G.B. (1997). N-acetylchitooligosaccharides elicit expression of a single (1-3)-b-glucanase gene in suspension-cultured cells from barley (Hordeum vulgare). – Physiol. Plant. 100: 111-118.
3. Kendra, D.F., Christian D., Hardwiger, L.A., (1989) oligomers from Fusarium Solani / pea interactions, ase / b-glucanase digestion of sporelings and from fungal wall actively inhibit fungal growth and enhance disease resistance. Physiological and Molecular Plant Pathology. 35: 215-230.
4. Organic Disease Control: patented (USA and International) Aeroponics International
See Assay Reports
See Related Information
Biocontrol info:
BEYOND All Natural Plant Amendment™ Brochure
Purchase ODC / Beyond Biocontrols and Aeroponic Units and Systems
Space Technology Food Production Reference Page
CSU Pre-flight Elicitor Assay Overviews - 1997![]()
Atlantis Launched to MIR Space Station (September 25th)
Purchase ODC / Beyond Biocontrols and Aeroponic Units and Systems
Use natural biocontrol products visit www.aeroponics.com